Jan 10
Chemistry Department Seminar: Tenure Track Candidate
"Enantioselective [2+2] Cycloadditions Using Visible Light Photoredox Catalysis"
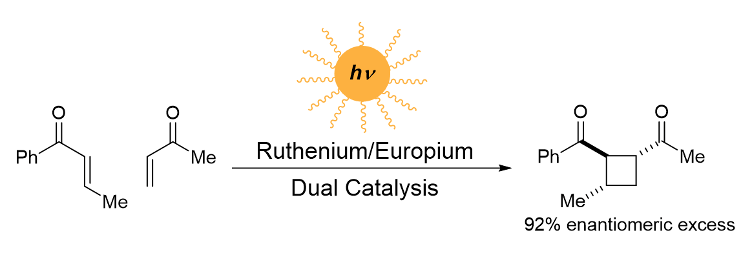
Photochemical reactions hold tremendous promise for synthetic chemistry. They are able to access mechanistic pathways that are unique from — and often complementary to — thermal reactions. However, synthetic photochemistry has traditionally been a niche field, in large part because of the need for specialized equipment and high-intensity UV light sources.
Our research focuses on photoredox catalysis, in which transition metal complexes absorb visible light, then use its energy to perform single electron redox chemistry with organic substrates. In our case, this enables intermolecular [2+2] cycloaddition reactions with mild light sources, sensitive functional groups, and acyclic substrates. Furthermore, our method is able to control the stereochemistry of these transformations, which has been a long-standing challenge in the field of synthetic photochemistry. Through the use of chiral lanthanide co-catalysts, we are able to convert achiral starting materials into chiral cyclobutane products with high diastereo- and enantioselectivity.
*This seminar counts towards the chemistry major seminar attendance requirement.
from Chemistry